2024 Volume 49 Issue 4 Pages 193-208
2024 Volume 49 Issue 4 Pages 193-208
Vascular endothelial cells serve as barriers between blood components and subendothelial tissue and regulate the blood coagulation-fibrinolytic system. Ionizing radiation is a common physical stimulant that induces a bystander effect whereby irradiated cells influence neighboring cells through signalings, including purinergic receptor signaling, activated by adenosine 5′-triphosphate (ATP), adenosine 5′-diphosphate (ADP), and adenosine as secondary soluble factors. Human vascular endothelial EA.hy926 cells were cultured and irradiated with γ-rays or treated with ATP, ADP, or adenosine under non-toxic conditions. RNA-seq, gene ontology, and hierarchical clustering analyses were performed. The transcriptome analysis of differentially expressed genes in vascular endothelial cells after γ-ray irradiations suggests that the change of gene expression by γ-irradiation is mediated by ATP and ADP. In addition, the expression and activity of the proteins related to blood coagulation and fibrinolysis systems appear to be secondarily regulated by ATP and ADP in vascular endothelial cells after exposure to γ-irradiation. Although it is unclear whether the changes of the gene expression related to blood coagulation and fibrinolysis systems by γ-irradiation affected the increased hemorrhagic tendency through the exposure to γ-irradiation or the negative feedback to the activated blood coagulation system, the present data indicate that toxicity associated with γ-irradiation involves the dysfunction of vascular endothelial cells related to the blood coagulation-fibrinolytic system, which is mediated by the signalings, including purinergic receptor signaling, activated by ATP and ADP.
Cardiovascular diseases (including cerebrovascular and ischemic heart diseases) cause a substantial degree of mortality globally, accounting for approximately one-third of all deaths worldwide (World Health Organization, 2020). Mortality from cardiovascular diseases continues to increase as a result of key modifiable risk factors, including an unhealthy diet, smoking, obesity, diabetes, hypertension, and rapid population aging (Zhao, 2021). Atherosclerosis is an inflammatory disease that provides the underlying pathology for cardiovascular diseases, and its onset and progression are attributed to the dysfunction of the vascular endothelial cells that cover the luminal surfaces of blood vessels (Ross, 1999).
Vascular endothelial cells serve as a barrier between blood components and subendothelial tissue and play a crucial role in maintaining vascular function through the production and release of various physiologically active substances. The antithrombotic properties of the cells are essential for blood circulation, and the dysfunction of blood coagulation and the fibrinolytic system can lead to the onset and progression of vascular diseases, including myocardial infarction and stroke. The fibrinolytic system instigates fibrin degradation by plasmin and is regulated by the balance between the tissue-type plasminogen activator and its inhibitor, plasminogen activator inhibitor-1 (Levin and Loskutoff, 1982).
In general, the risk of disease onset exhibits variability in human diseases and is determined by the interaction between genetic and environmental factors, which vary depending on the disease. The World Health Organization (2018) estimates that 30% of cardiovascular diseases are caused by chemical, physical, and physiological environmental factors. Chemical environmental factors including cadmium (Yamamoto et al., 1993; Hara et al., 2021), lead (Kaji et al., 1992a), arsenite (Nakano et al., 2021), and a copper complex (Fujie et al., 2017), while physiological environmental factors, including fibroblast growth factor-2 (Kaji et al., 1994), proteinase-activated receptor-1 (Yamamoto et al., 2005), endothelin-1 (Kaji et al., 1992b), calcium (Yamamoto et al., 1994), and transforming growth factor beta (Laiho et al., 1987), influence the fibrinolytic system in vascular endothelial cells. However, the effects of physical and environmental factors on endothelial fibrinolysis remain unclear.
Ionizing radiation is a typical physical stimulant that causes cellular injury, such as DNA damage and the production of reactive oxygen species (Ping et al., 2020). Exposure to ionizing radiation induces a bystander effect whereby irradiated cells influence neighboring cells. This process is mediated by purinergic receptor signaling, nucleotides, including adenosine 5′-triphosphate (ATP), adenosine 5′-diphosphate (ADP), and adenosine, as secondary soluble factors that are released from the irradiated cells to neighboring cells where they regulate cellular functions by stimulating purinergic receptors and downstream signals (Tsukimoto, 2015). In the present study, we investigated the comprehensive gene expression in vascular endothelial cells after exposure to γ-ray and evaluated the correlation with gene expression mediated by ATP, ADP, and adenosine using transcriptome analysis to elucidate the potential influence of ionizing radiation on vascular endothelial cellular function, particularly that of blood coagulation and fibrinolytic systems.
Human vascular endothelial EA.hy926 cells were purchased from ATCC (Manassas, VA, USA), Dulbecco’s modified Eagle’s medium (DMEM) and calcium- and magnesium-free phosphate-buffered saline (CMF-PBS) from Nissui Pharmaceutical (Tokyo, Japan), and fetal bovine serum (FBS), 12-well culture plates, and 24-well culture plates from Thermo Fisher Scientific (Waltham, MA, USA). The culture dishes and 96-well culture plates were purchased from NIPPON Genetics (Tokyo, Japan), ATP and ADP from Sigma-Aldrich (St. Louis, MO, USA), adenosine and 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) from FUJIFILM Wako Pure Chemical Industries (Osaka, Japan), May-Grünwald and Giemsa staining solutions from Merck KGaA (Darmstadt, Germany), and ISOGEN II from Nippon Gene (Tokyo, Japan). All the other reagents were purchased from Nacalai Tesque (Kyoto, Japan).
Cell culture and irradiationThe vascular endothelial cells were cultured at 37°C in 5% carbon dioxide in DMEM supplemented with 10% FBS until confluence. The medium was removed and the cells were washed twice with serum-free DMEM. The cells were then incubated in the presence or absence of ATP, ADP, or adenosine (0.1, 0.2, 0.5, or 1 mM) for 3 hr, 12 hr, or 24 hr in serum-free DMEM in 12-well or 24-well culture plates. Separately, vascular endothelial cells cultured in 12-well culture plates or 24-well culture plates were irradiated with γ-ray using a Gammacell 40 (137Cs source, 0.67 Gy/min) (Nordion International Inc., Ottawa, Canada) at room temperature for the specified time. After irradiation, the cells were incubated at 37°C in 5% carbon dioxide for 12 or 24 hr.
Cytotoxicity assayThe vascular endothelial cells were cultured in 24-well culture plates in the presence or absence of ATP, ADP, or adenosine (0.1, 0.2, 0.5, or 1 mM) for 24 hr in serum-free DMEM. Separately, vascular endothelial cells cultured in 24-well culture plates were irradiated with γ-rays (1, 2, 5, 10, or 20 Gy) and incubated for 24 hr in serum-free DMEM. After incubation, the cell layer was incubated for 1 hr in 100 µL fresh DMEM containing 0.25 mg/mL MTT. The DMEM was removed, and the cell layer was washed twice with CMF-PBS, and then lysed with 100 µL of dimethyl sulfoxide, after which the absorbance of the lysate at 570 nm was measured as a marker of cell viability using an ARVO Multimode Plate Reader (Perkin Elmer, Waltham, MA, USA).
RNA isolationThe vascular endothelial cells were cultured in 12-well plates in the presence or absence of 1 mM ATP, ADP, or adenosine for 3 or 12 hr in serum-free DMEM. Separately, vascular endothelial cells cultured in 12-well culture plates were irradiated with γ-ray at 20 Gy and then incubated for 12 hr in serum-free DMEM. After incubation, the conditioned medium was discarded, and the cell layer was lysed with 200 μL of ISOGEN II. Deionized distilled water (80 μL) was mixed with the lysate, and the mixture was centrifuged at 15,000 × g for 15 min. The supernatant was harvested, mixed with the same volume of 2-propanol, and centrifuged at 15,000 × g for 10 min; the supernatant was discarded. The precipitate was resuspended in 70% ethanol, centrifuged at 12,000 × g for 5 min, and dried. The quantity and quality of total RNA in the precipitate were assessed using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific).
RNA-seq analysisThe following procedures including library preparation, quality control analysis, and transcript assembly were outsourced to Macrogen (Seoul, Korea). Briefly, total RNA was subjected to library preparation for RNA-seq using the TruSeq RNA Sample Preparation Kit (Illumina, San Diego, CA, USA). After reverse transcription of the fragmented RNA into cDNA and amplification of the fragments by PCR, fragments with insert sizes of 200-400 bp were selected. The libraries were subsequently subjected to RNA-seq using a NovaSeq 6000 (Illumina), with conditions maintained at 101 cycles and paired-end sequencing. The quality of the FASTQ read data was checked using FASTQC v0.11.7 (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). The bases of reads that did not qualify for window size 4 using the sliding window method, had a mean quality score of 15 or lower, or lengths shorter than 36 bp were dropped to produce trimmed data using Trimmomatic 0.38 (http://www.usadellab.org/cms/?page=trimmomatic). The total number of filtered reads for each sample exceeded 45 million. The processed data were mapped to the reference genome GRCh38.p13 (Homo sapiens Assembly: GCF_000001405.39) using HISAT2 version 2.1.0, Bowtie2 (version 2.3.5.1) (https://ccb.jhu.edu/software/hisat2/index.shtml). Transcripts were assembled and annotated and expression levels were calculated using StringTie 2.1.3b (https://ccb.jhu.edu/software/stringtie/). Read count data were normalized using the trimmed mean of M-values (TMM) method in the EdgeR library. The false discovery rate (FDR) was used to correct the p values. Transcriptome data with p values of less than 0.05 and fold changes ≥ 3 were identified as statistically significant differentially expressed genes.
Gene ontology (GO) analysisThe list of differentially expressed genes with a p < 0.05 and fold change ≥ 3 was subjected to gene ontology (GO) analysis using the Database for Annotation, Visualization, and Integrated Discovery (DAVID) ver.2021 (https://david.ncifcrf.gov/) and Kyoto Encyclopedia of Genes and Genomes (KEGG, https://www.genome.jp/kegg/pathway.html). The GO terms in biological processes and KEGG pathways having p values < 0.05 were defined as significantly enriched gene clusters.
Hierarchical clustering analysisHierarchical clustering analysis of the patterns of the differentially expressed genes was performed using Heatmapper (http://www.heatmapper.ca/) using the following categories: clustering method, average linkage, distance measurement method, and Euclidean distance. Yellow and blue gradients indicate increases and decreases in gene expression, respectively.
Statistical analysisThe data shown in Figures 1B, 1C, and 1D were analyzed for statistical significance using analysis of variance (ANOVA) and Bonferroni-type multiple t-tests for multiple comparisons using Statcel3 (OMS, Tokyo, Japan).
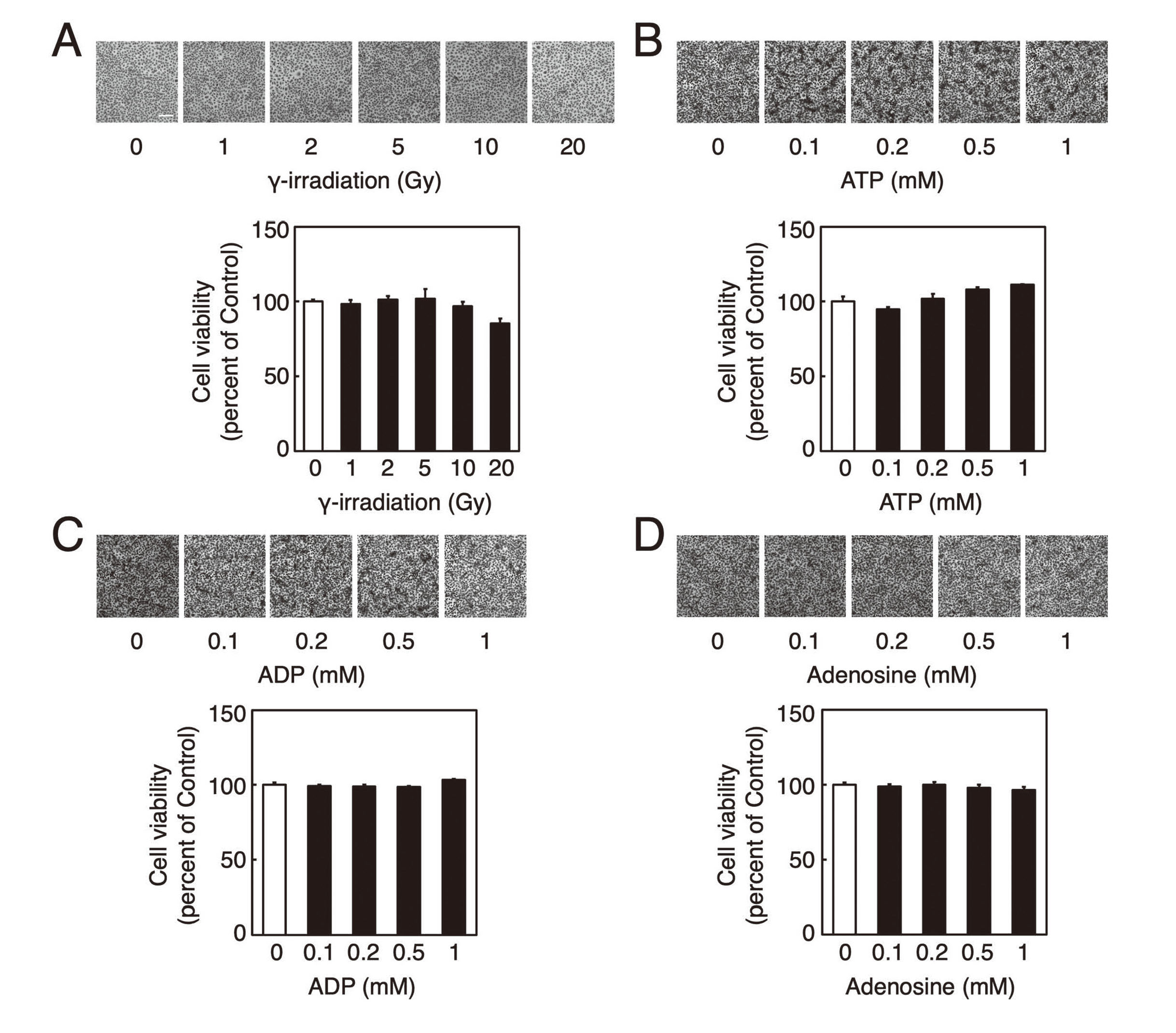
Cytotoxicity of γ-irradiation, ATP, ADP, and adenosine in vascular endothelial cells. The vascular endothelial cells were [A] irradiated with γ-ray at 1, 2, 5, 10, or 20 Gy and then incubated for 24 hr, or [B, C, D] incubated in the presence or absence of 0.1, 0.2, 0.5, or 1 mM of ATP (B), ADP (C), or adenosine (D) for 24 hr. After incubation, the cell layer was stained with Giemsa and cell viability was measured by MTT assay. Original magnification (×40). Scale bar = 250 μm. Values are means ± SE of four independent samples.
The investigation of the damage to the cultured vascular endothelial cells by γ-ray irradiation, ATP, ADP, and adenosine revealed no cytotoxicity after irradiation with γ-ray at ≤ 20 Gy and the viability of the cells was not significantly decreased (Fig. 1A). In addition, cytotoxicity was not observed in the vascular endothelial cells after exposure to ATP, ADP, or adenosine at concentrations of ≤ 1 mM (Fig. 1B, 1C, and 1D). Thus, a transcriptomic analysis was performed at an irradiation dose of 20 Gy, and ATP, ADP, and adenosine concentrations of 1 mM.
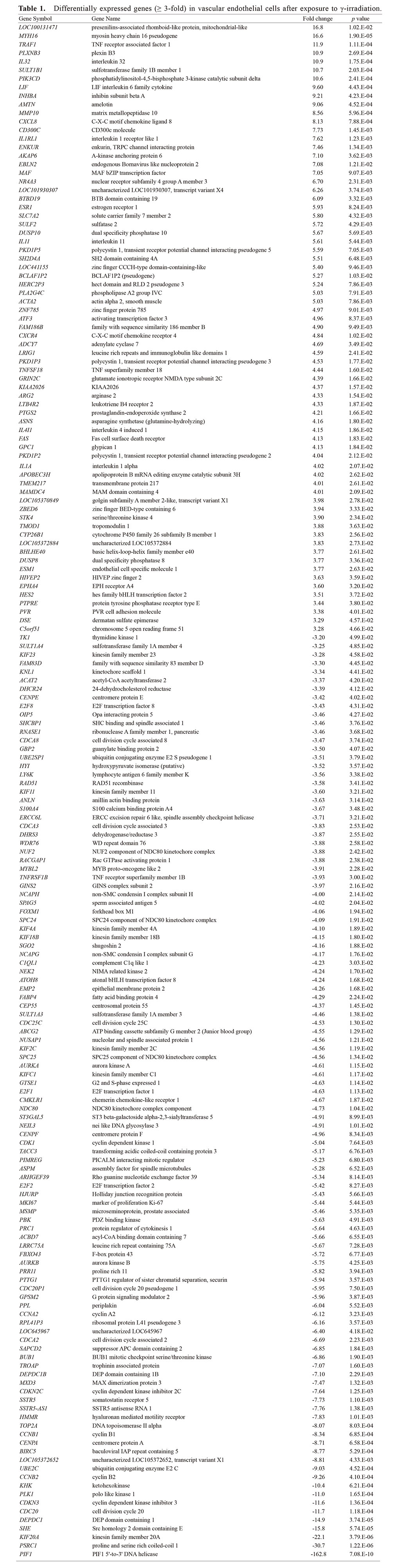
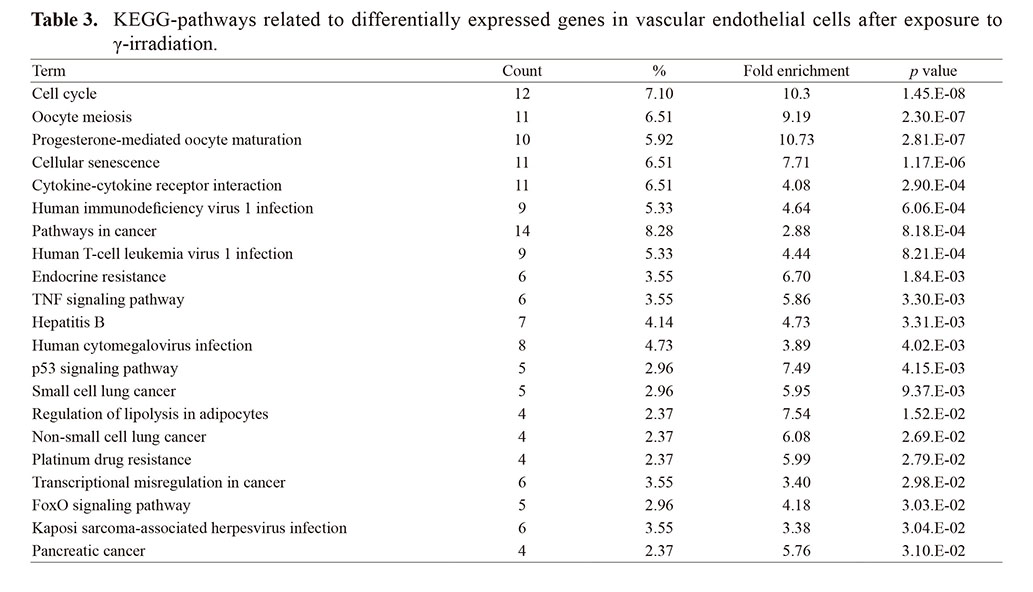
The vascular endothelial cells were irradiated with γ-ray for 12 hr and total RNA samples were extracted. We identified 15,263 differentially expressed genes in at least two samples on the RNA-seq analysis. A total of 179 differentially expressed genes (172 annotated and 7 non-annotated) were significantly identified in the γ-irradiated samples; 71 genes were expressed by more than 3-fold and the remaining 108 were decreased more than 3-fold compared to the control (Table 1). These 179 differentially expressed genes were applied to GO biological process (Table 2) and were found to be related to the following four terms: “cell cycle” (56 genes, 33.1%), “cell division” (48 genes, 28.4%), “mitosis” (45 genes, 26.6%), and “chromosome partition” (7 genes, 4.14%). Irradiation with γ-ray induces DNA damage and its repair response, arrests the cell cycle at the G1 phase, and promotes proinflammatory responses in vascular endothelial cells (Baselet et al., 2017; Ungvari et al., 2013; Schröder et al., 2019), and the identified GO terms reflect these DNA damage and repair responses. In addition, these 179 differentially expressed genes were applied to a DAVID pathway analysis (Table 3), which indicated that the following 21 terms were significantly related: five cell cycle terms (Cell cycle, Oocyte meiosis, Endocrine resistance, p53 signaling pathway, and FoxO signaling pathway), two inflammatory response terms (Cytokine-cytokine interaction and TNF signaling pathway), five infection related-terms (Human immunodeficiency virus 1 infection, Human T-cell leukemia virus 1 infection, Hepatitis B, Human cytomegalovirus infection, and Kaposi sarcoma-associated herpesvirus infection), and six cancer-related terms (Pathways in cancer, Small cell lung cancer, Non-small cell lung cancer, Platinum drug resistance, Transcriptional misregulation in cancer, and Pancreatic cancer).

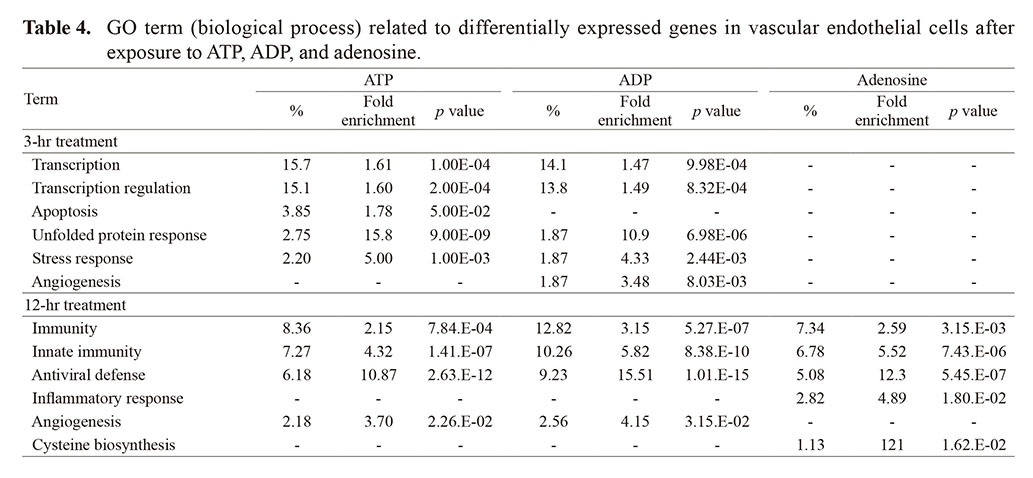
We then investigated the changes in gene expression after the vascular endothelial cells were subjected to 3 hr of ATP, ADP, and adenosine exposure. The samples revealed 386, 447, and 208 differentially expressed genes, respectively, whereas 309, 205, and 195 differentially expressed genes were identified in the samples subjected to 12 hr of ATP, ADP, and adenosine exposure, respectively (data not shown). When these differentially expressed genes were classified using GO terms of biological processes, the differentially expressed genes in the samples exposed to ATP for 3 hr were significantly related to the following five terms: “Transcription” (57 genes, 15.7%), “Transcription regulation” (55 genes, 15.1%), “Apoptosis” (14 genes, 3.85%), “Unfolded protein response” (10 genes, 2.75%), and “Stress response” (8 genes, 2.20%) (Table 4). Of these, four were found in the samples subjected to ADP exposure for 3 hr (Unfolded protein response, Transcription, Transcription regulation, and Stress response), whereas none were significantly identified in the samples subjected to adenosine exposure for 3 hr. The 3-hr ADP sample identified “Plasminogen activation” (2 genes, 0.47%), but not significantly (p = 0.0080, data not shown), while the 12-hr ATP sample detected differentially expressed genes that were significantly related to the following four terms: “Immunity” (23 genes, 8.36%), “Innate immunity” (20 genes, 7.27%), “Antiviral defense” (17 genes, 6.18%), and “Angiogenesis” (6 genes, 2.18%), which were common to the 12-hr ADP sample, and the initial three terms were also common to the 12-hr adenosine sample. Terms such as immune response and angiogenesis were consistent with previous reports on the regulation of cellular functions in vascular endothelial cells by ATP (von Albertini et al., 1998; Kaczmarek et al., 2005). Overall, genes related to transcription and stress response were changed by the innate response to ATP and ADP exposure, whereas the genes related to immune response, inflammation, cancer, and viral infection showed a delayed response to ATP, ADP, and adenosine exposure in vascular endothelial cells. However, none of the terms observed in the ATP, ADP, and adenosine samples were consistent with those in the γ-irradiation sample. This lack of overlap is likely due to the absence or reduced influence of the bystander effect by nucleotides because of the relatively short incubation time after γ-irradiation in the present study. These results could indicate that the secondary effects of nucleotides are crucial for regulating cellular function, including immune response and angiogenesis, in vascular endothelial cells after γ-ray irradiation. The changes in gene expression after ATP or ADP exposure were similar, and subsequently, those of adenosine exposure resembled those of ATP and ADP in the vascular endothelial cells. ATP and ADP bind to P2 receptors that are classified as ionotropic P2X and metabotropic P2Y receptors, whereas adenosine binds to G protein-coupled adenosine (P1) receptors (Kukulski et al., 2011). Extracellular ATP and ADP are converted into AMP by CD39, ecto-nucleoside triphosphate diphosphohydrolase 1, and AMP is de-phosphorylated into adenosine by CD73, an ecto-5'-nucleotidase, on the plasma membrane (Antonioli et al., 2013). These results suggest that the regulation of gene expression by ATP and ADP differed from that by adenosine because of the differences in the target receptors and that the effects of ATP and ADP may be partially associated with adenosine degradation due to the extracellular metabolisms of ATP and ADP related to CD39 and CD73.
The differentially expressed genes in the 3-hr ATP sample were applied to DAVID pathway analysis (Table 5), which identified the following 16 significantly related pathways: seven related to immune and inflammatory responses (Cytokine-cytokine receptor interaction, TNF signaling pathway, TGF-beta signaling pathway, IL-17 signaling pathway, Rheumatoid arthritis, NF-kappa B signaling pathway, and JAK-STAT signaling pathway), three related to stress responses (Protein processing in endoplasmic reticulum, Lipid and atherosclerosis, and AGE-RAGE signaling pathway in diabetic complications), two related to infections (Viral protein interaction with cytokine and cytokine receptor, and Malaria), two related to ribosomes (Ribosome biogenesis in eukaryotes, and Ribosome), two related to cellular proliferation and survival (Signaling pathways regulating pluripotency of stem cells and MAPK signaling pathway), and one related to cancer (Transcriptional misregulation in cancer). Four of these terms were common to the 3-hr ADP and adenosine samples and were signals related to the inflammatory response (TNF signaling pathway, TGF-beta signaling pathway, IL-17 signaling pathway, and NF-kappa B signaling pathway). Furthermore, four terms in the ATP sample were consistent with those in the γ-irradiated sample (Cytokine-cytokine receptor interaction, TNF signaling pathway, Transcriptional misregulation in cancer, and FoxO signaling pathway), suggesting that the activation of these signals in the vascular endothelial cells after γ-ray irradiation were mediated by ATP and ADP.
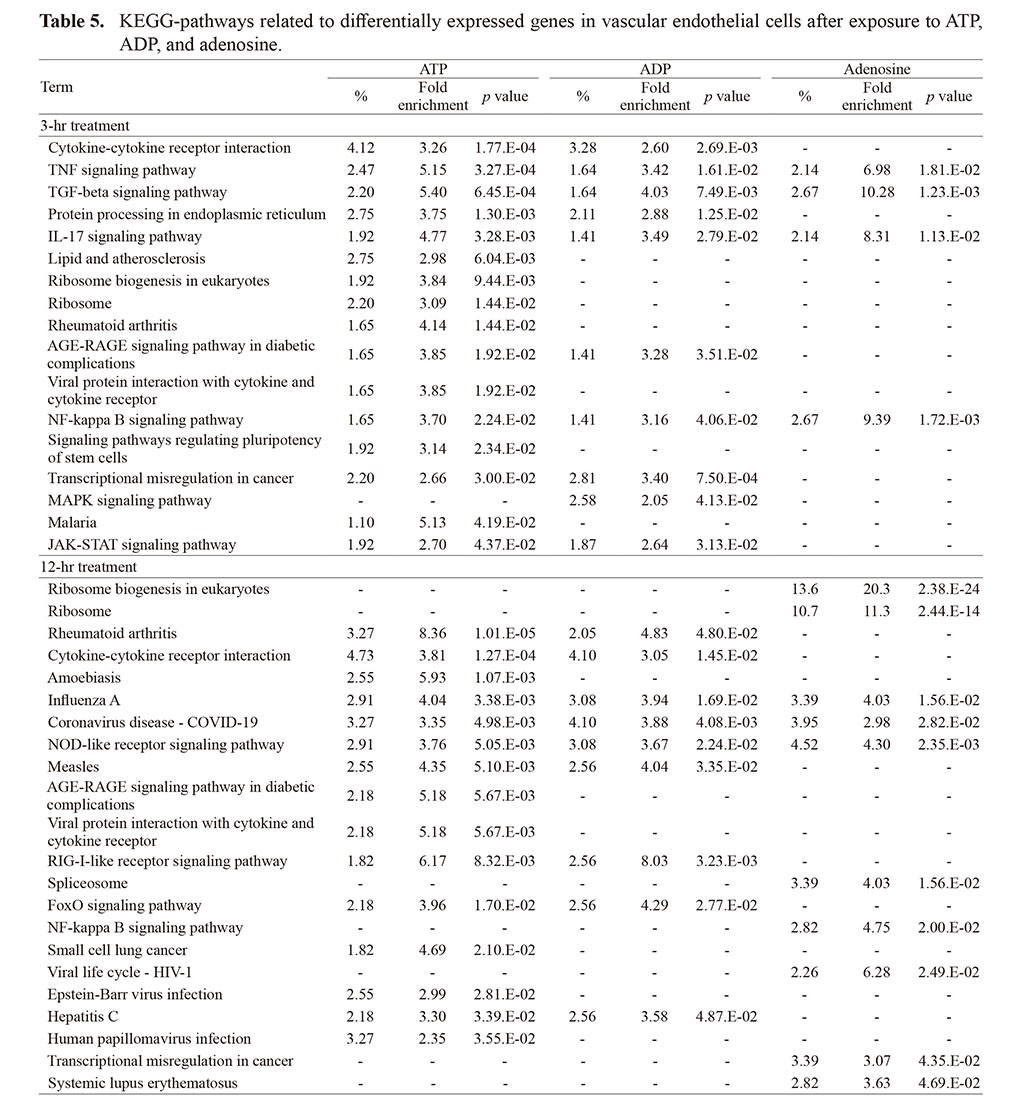
To determine whether the 179 genes that were differentially expressed by γ-irradiation were related to ATP, ADP, and the adenosine-mediated signaling pathway, we performed a correlation analysis between the differentially expressed genes in the γ-irradiation sample and those in the ATP, ADP, and adenosine samples. The cluster analysis showed that the pattern of differentially expressed genes in the γ-irradiation sample was similar to those in the ATP and ADP samples, and the trends were more marked than those in the adenosine sample (Fig. 2A). Using regression analysis (Fig. 2B), the differentially expressed genes in the γ-irradiation sample exhibited a significant correlation with those in the 3-hr ATP and ADP samples (p = 0.0005 and 0.0006, respectively). Similarly, significant correlations were observed with the 12-hr ATP and ADP samples (p = 0.0030 and 0.0181, respectively), although to a lesser degree than those with the 3-hr samples. In contrast, the differentially expressed genes in the 3-hr and 12-hr adenosine samples were not significantly correlated with those in the γ-irradiated sample (p = 0.8171 and 0.0921, respectively), suggesting that the changes of gene expression by γ-irradiation were mediated by ATP and ADP, but not adenosine, in the vascular endothelial cells.

Comparison between γ-irradiation and adenine metabolites for identified differentially expressed genes. [A] Hierarchical clustering analysis for 179 differentially expressed genes identified in the cultured vascular endothelial cells after exposure to γ-irradiation, ATP, ADP, or adenosine. The vascular endothelial cells were irradiated with γ-ray (20 Gy) and then incubated for 12 hr, or incubated in the presence or absence of ATP, ADP, or adenosine (1 mM each) for 3 or 12 hr. Increased genes are represented in yellow, while decreased genes are represented in blue. [B] Scatterplots of the 179 differentially expressed genes identified in the cultured vascular endothelial cells after exposure to γ-ray, ATP, ADP, or adenosine. The vascular endothelial cells were subjected to γ-irradiation (20 Gy) and then incubated for 12 hr, or incubated in the presence or absence of ATP, ADP, or adenosine at 1 mM for 3 or 12 hr. Coefficients of determination and p values are provided for all scatter plots.
Finally, the γ-irradiated, ATP, ADP, and adenosine samples and their correlation were investigated regarding the differentially expressed genes related to the GO term “blood coagulation”. This term was found to be directly associated with 80 genes, with 41 of these slightly changed in the γ-irradiated sample, although no significant changes were observed (Table 6). In both the 3-hr and 12-hr ATP and ADP samples, the expression of three genes (TFPI2, SERPIND1, and PLAT) was increased, whereas the expression of HGFAC and F2RL3 were increased and decreased, respectively, in the 3-hr and 12-hr adenosine samples (Table 7). TFPI2 encodes Tissue factor pathway inhibitor 2, which is a serine protease inhibitor of the Tissue factor-VIIa complex, activated factor XI, plasmin, and certain matrix metalloproteinases (Crawley et al., 2002). Similarly, SERPIND1 encodes Heparin cofactor II, a protease inhibitor that is produced in the liver to inactivate thrombin in the plasma by forming the thrombin-heparin cofactor II complex (Tollefsen, 2010). PLAT is a gene responsible for encoding the tissue-type plasminogen activator, which plays a crucial role in activating the fibrinolysis system (Levin and Loskutoff, 1982). HGFAC encodes the Hepatocyte growth factor activator, a coagulation factor XII-like serine endopeptidase that activates a activator for hepatocyte growth factor (Fukushima et al., 2018). Hepatocyte growth factor induces the expression of PAI-1 and tissue factor in hepatocellular carcinoma-derived HepG2 cells, although such induction does not occur in human umbilical vein endothelial cells (Wojta et al., 1994). F2RL3 encodes Protease-activated receptor 4, a transmembrane G protein-coupled receptor for thrombin, which controls platelet aggregation through the help of Protease-activated receptors 1, 2, and 3 (Fu et al., 2015). The cluster analysis showed that the pattern of the 41 differentially expressed genes associated with the GO term “blood coagulation” in the γ-irradiation sample was similar to those in the ATP and ADP samples, which were more marked than that in the adenosine sample (Fig. 3A). The regression analysis (Fig. 3B) revealed that the 41 differentially expressed genes in the γ-irradiation sample exhibited significant correlations with those from the samples exposed to ATP and ADP for 3 hr (p = 0.0011 and 0.0014, respectively), and this significant correlation was also observed with the 12-hr ATP and ADP samples (p = 0.00003 and 0.00003, respectively). However, those in the 3-hr and 12-hr adenosine samples were not significantly correlated with those in the γ-irradiation sample (p = 0.1246 and 0.3112, respectively), suggesting that the changes of the gene expression associated with the GO term “blood coagulation” by γ-irradiation in the vascular endothelial cells were mediated by ATP and ADP, but not that of adenosine. Therefore, the changes in blood coagulation and the fibrinolytic systems in the vascular endothelial cells after γ-ray irradiation are likely mediated by the signalings activated by ATP and ADP.

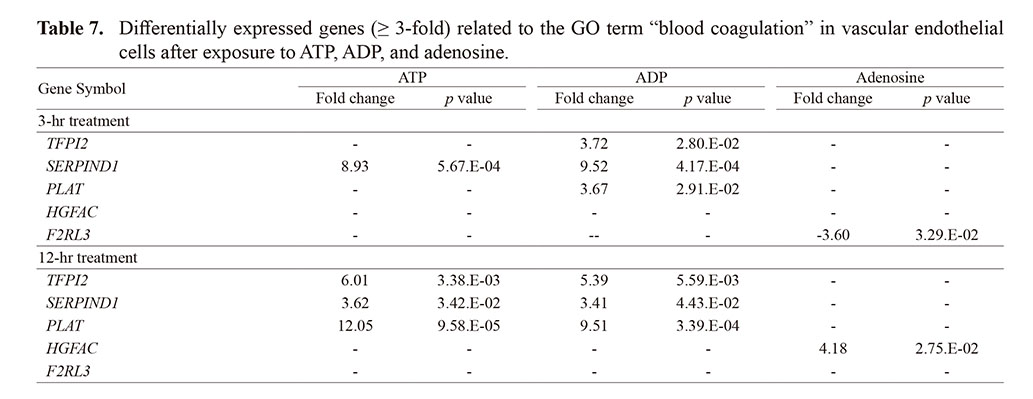
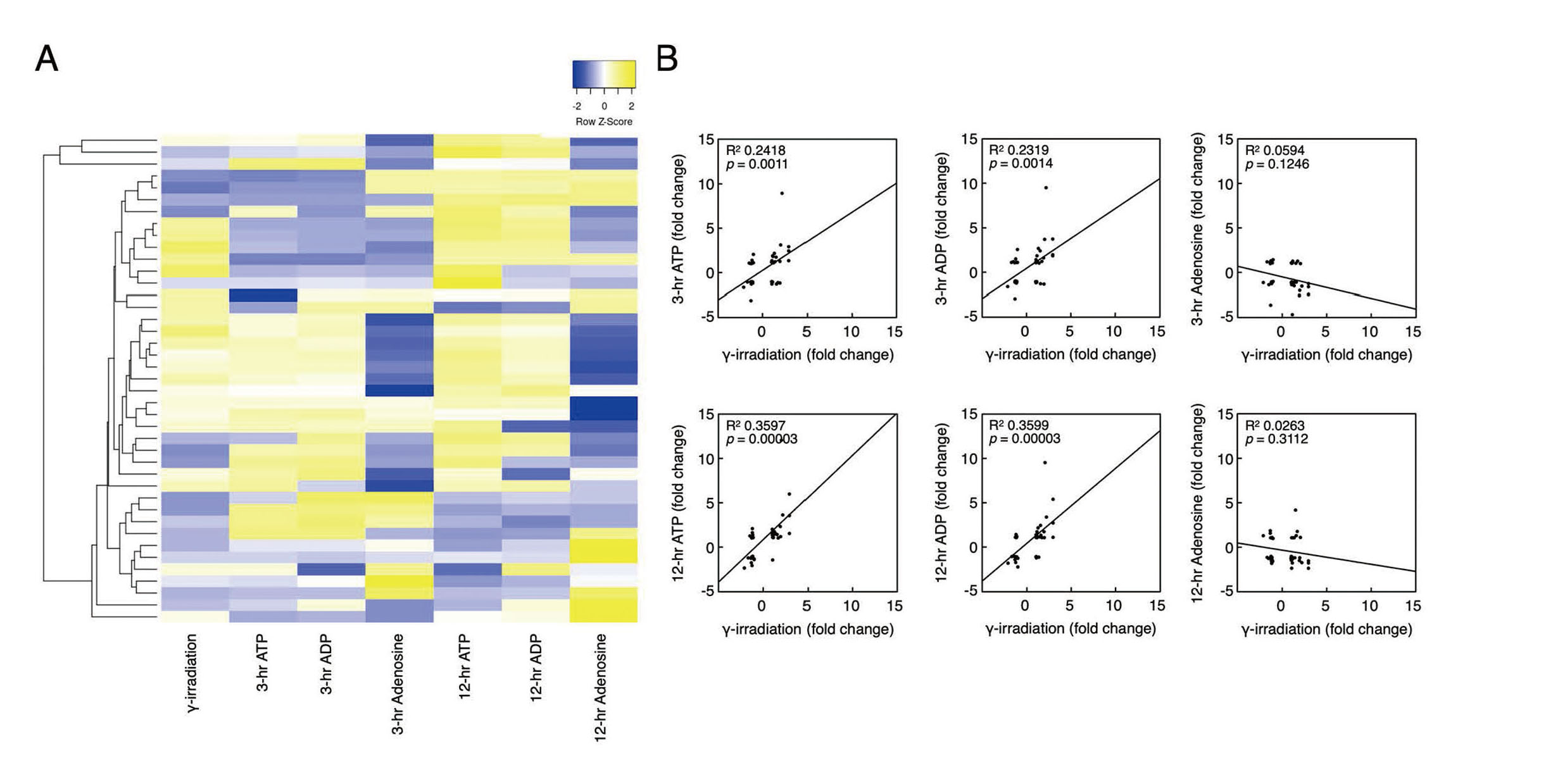
Comparison of γ-irradiation and adenine metabolites for identified differentially expressed genes related to the GO term “blood coagulation.” [A] Hierarchical clustering analysis for 40 differentially expressed genes related to the GO term “blood coagulation” identified in cultured vascular endothelial cells after exposure to γ-irradiation, ATP, ADP, or adenosine. The vascular endothelial cells were subjected to γ-irradiation (20 Gy) and then incubated for 12 hr, or incubated in the presence or absence of ATP, ADP, or adenosine (1 mM each) for 3 or 12 hr. Increased genes are represented in yellow, while decreased genes are represented in blue. [B] Scatterplots of the 40 differentially expressed genes related to the GO term “blood coagulation” identified in the cultured vascular endothelial cells after exposure to γ-irradiation, ATP, ADP, or adenosine. The vascular endothelial cells were subjected to γ-irradiation (20 Gy) and then incubated for 12 hr, or incubated in the presence or absence of ATP, ADP, or adenosine at 1 mM for 3 or 12 hr. Coefficients of determination and p values are provided for all scatter plots.
In the present study, the transcriptome analysis of differentially expressed genes in vascular endothelial cells suggests that the γ-ray-induced change of gene expression was mediated by the signalings activated by ATP and ADP and that the expression and activity of the proteins related to blood coagulation and fibrinolysis systems were secondarily regulated by the signalings after exposure of vascular endothelial cells to γ-irradiation (Fig. 4). Since an epidemiological study suggested that ionizing radiation at 1 Gy increases the incidence of atherosclerosis and cardiovascular diseases (Darby et al., 2013), the mechanisms underlying vascular endothelial cell damage and its response to ionizing radiation are important. Although the gene expression associated with the GO term “blood coagulation” tended to inhibit the blood coagulation system and activate the fibrinolytic system, the response of the vascular endothelial cells to γ-irradiation and nucleotides was based on a limited time measurement. Therefore, it is unclear whether the changes of the gene expression related to the blood coagulation and fibrinolysis systems by γ-irradiation were reflected in the promotion of the hemorrhagic tendency by exposure to γ-irradiation or the negative feedback to the activated blood coagulation system. However, the present data indicate that the toxicity of γ-irradiation causes the dysfunction of vascular endothelial cells related to the blood coagulation-fibrinolytic system, which is mediated by the signalings activated by ATP and ADP. Further studies are required to understand the gene expression, activity, and mechanisms underlying this dysfunction of the coagulation and fibrinolysis systems by γ-irradiation in detail.

Schematic of possible mechanisms underlying the regulation of the blood coagulation-fibrinolytic system in γ-irradiation in vascular endothelial cells. When the vascular endothelial cells are irradiated with γ-ray, ATP and ADP released from the cells activate the purinergic receptor signaling. The blood coagulation-fibrinolytic system is regulated by ATP- and ADP-mediated signalings, including the purinergic receptor signaling, and other signalings activated by γ-irradiation in the vascular endothelial cells.
We would like to thank Editage (www.editage.com) for English language editing.
Conflict of interestThe authors declare that there is no conflict of interest.